Trump Tariffs: Impact & Opportunities in Indian Pharma
Indian pharma is exempt from Trump’s 2025 tariffs — for now. Specialty & biosimilar firms hold strong, but generics-heavy players face margin, regulatory risk.
Table of Content
Impact of Trump’s Reciprocal Tariffs on Indian Pharma Industry
Indian Pharma Industry’s Strategic Response to Tariff Risks
Hidden Growth Opportunities for Indian Pharma Amid Trade Tensions
Winners & Losers in Indian Pharma Post-Tariffs
1. Impact of Trump’s Reciprocal Tariffs on Indian Pharma Industry
1.1 Current Tariff Status: Indian Pharma Temporarily Exempt
Pharmaceutical products (formulations, APIs) are currently excluded from the 26% tariffs imposed by the U.S. on a wide range of Indian imports.
However, Trump has previously signaled discomfort with low-cost generics flooding the U.S. market and has pushed for “Buy American” healthcare policies.
Implication: The exemption may be temporary or politically reversible. Future tariffs, restrictions, or non-tariff actions are very possible.
1.2 U.S. Dependency of Indian Pharma Exports
The U.S. is the #1 market for Indian generics, with annual exports > $10 billion
The U.S. accounts for ~30-35% of India’s pharma exports, particularly in generic formulations.
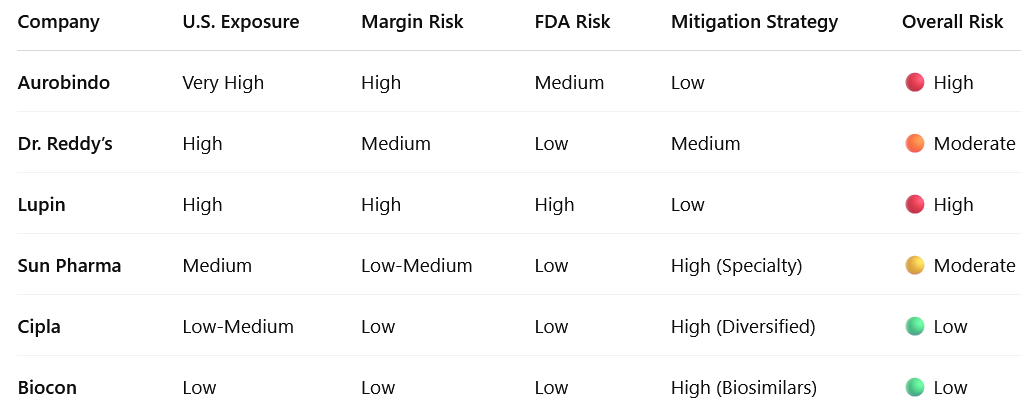
Leading exporters like Aurobindo, Dr. Reddy’s, Lupin, and Sun Pharma derive a major chunk of revenues from the U.S. generics market.
Implication: Even indirect disruptions (e.g., lower demand from U.S. buyers or tighter regulation) can significantly affect toplines.
Risk Level: High for generics-heavy exporters with >35% U.S. share
1.3 Tariffs and Margin Risk in Indian Generic Drugs
Generic drugs in the U.S. already operate on razor-thin margins due to:
Intense price competition
Buyer consolidation (e.g., Walgreens, CVS, Cardinal, McKesson)
Regulatory costs
A 10–15% price hit from tariffs could wipe out margins on many low-value generics.
Many Indian pharma players won’t be able to pass on cost increases
Companies with branded generics or specialty drugs (e.g., Sun Pharma’s Taro, Biocon’s biosimilars) are better insulated.
1.4 Regulatory Risk as a Non-Tariff Barrier
Even in the absence of direct tariffs, the U.S. administration may use non-tariff barriers such as:
Stricter FDA inspections
Delays in drug approvals
Increased scrutiny of Indian manufacturing facilities
Implication: Regulatory pressure can serve as a de facto tariff by slowing down business operations and adding compliance costs.
Impacted the most: Companies that rely on single-site filings or have prior FDA warnings (e.g., Lupin, Aurobindo have had issues in past)
1.5 Supply Chain Localization Pressure on Indian Pharma
U.S. policymakers are pushing for domestic manufacturing of critical drugs and APIs, citing supply chain security and strategic autonomy.
While Indian companies still dominate the generics market, there's growing U.S. interest in reshoring production.
Most Indian pharma companies do not have significant U.S. manufacturing bases. A few exceptions:
Sun Pharma: Taro Pharma (U.S.)
Cipla: Some capacity via acquisition (InvaGen)
Dr. Reddy’s: Limited, but exploring local alliances
Implication: Indian pharma faces long-term pressure to localize manufacturing, invest in U.S.-based facilities, or partner with American CDMOs.
1.6 Why Product Mix Will Define Pharma Winners & Losers
Biocon and Cipla are pivoting toward biosimilars, specialty inhalers, and value-added generics to escape the price war.
Sun Pharma is investing in dermatology, branded formulations, and specialty assets in the U.S.
Policy-wise, Indian pharma associations (like IPA, Pharmexcil) are lobbying for continued tariff exemption based on healthcare affordability arguments.
Signs of proactive adaptation, but not universal across the sector
High Risk:
Oral solids (tablets/capsules)
Off-patent, non-differentiated generics
Low-barrier-to-entry APIs
More Resilient:
Biosimilars and specialty drugs (e.g., injectables, oncology, respiratory)
Branded generics
CDMO/CRAMS services for global innovators
Implication: Firms moving up the value chain will better withstand global trade and regulatory shocks.
1.7 Trade Uncertainty & Impact on Pharma M&A Strategy
Trade uncertainty makes U.S. acquisition targets more expensive and riskier
Pharma partnerships with U.S. firms may slow down due to regulatory bottlenecks or nationalistic preference shifts
Indian companies may need to invest in U.S. facilities to hedge against future barriers
1.8 How Tariff Uncertainty Affects Pharma Valuations
Even if tariffs don’t materialize immediately, the fear of future barriers creates:
P/E multiple compression
Reduced foreign fund interest in U.S.-heavy pharma stocks
2. Indian Pharma Industry’s Strategic Response to Tariff Risks
The Indian pharma industry sees the Trump tariffs more as a diplomatic hurdle than a structural threat. They are confident in their competitive moat, cautious about making big reactive changes, and leaning on policy resolution while staying focused on long-term strategy.
2.1 Indian Pharma Sees Tariffs as Temporary, Not Structural
Most managements are not overreacting to the threat of tariffs. The consensus is that:
Tariffs are temporary and politically driven, not structural.
Business decisions—like setting up U.S. facilities—should not be reactionary.
"I'm not sure tariffs should dictate what we should be doing as players, because there is a risk that four years later, those tariffs may go away," Cipla Global CEO Umang Vohra
"I don't know how much difference it (tariffs) will make to us... and will not justify relocating our manufacturing," Sun Pharma MD Dilip Shanghvi.
2.2 Why Indian Pharma Remains Confident in Cost Competitiveness
There’s broad confidence that Indian generics will remain competitive, even with a 26% tariff:
Indian pharma has strong cost advantages and scale.
Dr. Reddy’s MD G.V. Prasad called out that relocating to the U.S. isn’t viable due to high costs and lack of infrastructure.
2.3 Will Tariffs Be Passed to U.S. Consumers?
Companies like Sun Pharma indicate that additional costs may be passed on, especially in non-competitive segments:
There's a belief that the U.S. still depends heavily on Indian generics.
Price increases are inevitable in some areas.
"Ultimately, it (tariff impact) will be passed on to consumers," Sun Pharma MD Dilip Shanghvi.
2.4 Role of Government Diplomacy in Defending Pharma Exports
The industry is collectively banking on government-to-government negotiations:
The Indian Pharmaceutical Alliance and major companies are coordinating with policymakers.
There's optimism that pharma will remain exempt or get preferential treatment due to its critical nature.
“I welcome the US government’s decision to exempt Indian pharmaceutical products from tariffs, which underscores the strong bilateral relationship between India and the US, as well as the critical role of the Indian pharmaceutical sector in enhancing public health,” Biocon Chairperson Kiran Mazumdar-Shaw
2.5 No Immediate Shift in Indian Pharma Manufacturing Strategy
Despite U.S. pressure for domestic manufacturing:
No major company is planning to shift supply chains or build new capacity in the U.S.
Indian players believe their current base can handle global demand efficiently.
“Building domestic manufacturing capacity to meet these needs will take years of investment, regulatory adjustments, and workforce development. It’s heartening to see our longstanding partnership with the US being recognized—this relationship has fostered an environment where open dialogue can lead to mutual growth.” Sheetal Arora, CEO & Promoter, Mankind Pharma
3. Hidden Growth Opportunities for Indian Pharma Amid Trade Tensions
3.1 India’s Advantage as an Alternative to Chinese API Suppliers
If U.S. keeps penalizing China (via tariffs, blacklists, or ESG filters), India becomes the default alternative for API sourcing
Opportunity: Gain share in paracetamol, antibiotics, anti-diabetics, oncology APIs
Beneficiaries: Divi’s, Laurus Labs, Aarti Drugs, Neuland
3.2 Biosimilars and Specialty Products: The Next Growth Engine
U.S. biotech and specialty pharma companies need cost-effective, trusted partners
India’s contract development & manufacturing sector (CRAMS/CDMO) is gaining traction fast
Opportunity: Long-term, high-margin partnerships
Beneficiaries: Syngene, Suven, Gland Pharma, Laurus
3.3 Growth in Biosimilars and Specialty Products
U.S. needs cost-efficient alternatives to biologics — but manufacturing and regulatory costs are high domestically
Indian firms with biosimilar pipelines can break into high-value, low-competition markets
Beneficiaries: Biocon Biologics, Dr. Reddy’s, Zydus Lifesciences
3.4 Nearshoring Manufacturing Hubs
These can serve U.S. buyers tariff-free and reduce geopolitical friction
Beneficiaries: Companies with capital flexibility and global compliance systems
3.5 PLI Scheme Driving Domestic Pharma Capex
India’s Production Linked Incentive (PLI) scheme is driving capex in:
Key APIs
Complex generics
Domestic manufacturing
Beneficiaries: Mid-tier players upgrading capabilities, especially in API backward integration
4. Winners & Losers in Indian Pharma Post-Tariffs
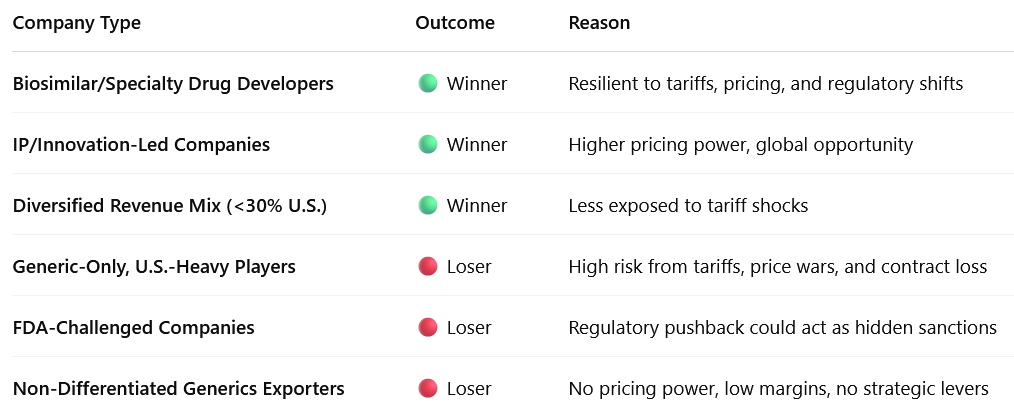
4.1 Winners: Specialty Drug Makers and Biosimilar Leaders
Why: These products are higher-margin, harder to commoditize, and less price-sensitive. U.S. payers and hospitals need them.
Traits:
Strong U.S. pipeline of inhalers, injectables, oncology, dermatology, etc.
Not reliant on high-volume, low-margin generics
Diversifying toward Europe and emerging markets
Examples (by model, not recommendation):
Biosimilar-focused: Biocon Biologics
Specialty-focused: Sun Pharma (via Taro, dermatology)
Respiratory play: Cipla (inhalers, complex generics)
4.2 Winners: Diversified Pharma Companies with Low U.S. Exposure
Why: Less exposed to direct or indirect policy shocks
Traits:
U.S. share <30% of total revenue
Balanced presence in EU, Africa, India, and RoW
Manufacturing flexibility outside India
Examples (by model):
Domestic-heavy: Torrent Pharma
Well-diversified: Cipla, Biocon
4.3 Winners: IP-Driven and R&D-Led Pharma Players
Why: Tariffs and pricing pressure hurt commodity businesses, not IP-led ones
Traits:
Strong R&D pipelines
Differentiated delivery platforms (e.g., nano-formulations, fixed-dose combos)
Partnerships with MNCs or U.S. healthcare systems
Examples (by model):
Niche R&D players: Syngene, Laurus (CDMO)
Global partnerships: Glenmark (with partners in EU/US)
4.4 Losers: Generics-Heavy, U.S.-Dependent Pharma Exporters
Why: Tariff hikes, buyer consolidation, and FDA risks hit this model hardest
Traits:
40–50% of revenue from U.S.
Portfolio dominated by oral solids and commoditized generics
History of FDA compliance issues
Risk Example (by model):
Aurobindo, Lupin, and other generics-heavy exporters
4.5 Losers: Pharma Firms with FDA or Compliance Challenges
Why: Trump-era FDA could be used as a non-tariff weapon (e.g., increased inspections, warning letters)
Traits:
Plants with ongoing or unresolved warning letters/import alerts
Heavy reliance on 1–2 FDA-approved sites
Reactive instead of proactive compliance
Implication: Disproportionate scrutiny, approval delays, and export disruption
4.6 Losers: Companies Without Innovation or Market Diversification
Why: Tariffs + price pressure + regulatory fog = death spiral for me-too generic companies
Traits:
No differentiated products or platforms
Poor visibility outside U.S. market
No strategic shift toward complex generics or branded exports
Disclaimer
Content Accuracy and Reliability: This summary of the earnings call is generated using an artificial intelligence large language model (LLM). While every effort has been made to ensure the accuracy and completeness of the information, the summary may not fully capture all nuances or details of the original earnings call. The content provided is for informational purposes only and should not be construed as financial advice or a recommendation to buy or sell any securities. Verification: Readers are encouraged to refer to the official earnings call transcript, company filings, and other authoritative sources for comprehensive and accurate information. The creators of this summary do not guarantee the accuracy, completeness, or timeliness of the information and accept no responsibility for any errors or omissions. No Liability: The use of this summary is at your own risk. The creators and distributors of this content disclaim any liability for any loss or damage arising from the use of or reliance on this summary. Consult Professional Advice: For investment decisions or financial advice, please consult a qualified financial advisor or other professional


We did a similar deep-dive. Good to see we are aligned on the big picture risks!
https://nordicedge.substack.com/p/patents-pills-and-protectionism-the